ECHA Public activities coordination tool
A new version of the Public activities coordination tool (PACT) has been integrated in the European Chemicals Agency (ECHA) website. This searchable and customizable database now includes substances for which dossier evaluation is ongoing and registries of CLP/restriction/SVHC intentions. With this revamped tool, the transparency of authorities’ activities has certainly increased.
The public activities coordination tool (PACT) has been developed by ECHA in order to track the substance-specific activities being undertaken under REACH and the CLP Regulations in line with its Integrated Regulatory Strategy. Together with the Member States, ECHA developed a common screening process, which identifies substances with adverse impacts on human health and the environment, while prioritizing substances for further compliance check, evaluation or EU level risk management measures.
Under the compliance check process, priority is given to chemicals produced over 100 tonnes per year. The main focus is on the higher tier human health and environmental endpoints relevant for identifying CMR (carcinogenic, mutagenic and reprotoxic) and PBT/vPvB ((very) persistent, bioaccumulative and toxic) substances. Inclusion in this searchable list means that the substance is under examination by a Member State or ECHA. If the suspected properties are concluded upon evaluation, further regulatory risk management actions will be imposed after the relevant regulatory list – such as the Candidate List, the restricted substances list, or Annex VI to CLP is updated.
The chart below displays how the activities and regulatory processes relate to each other. It also shows the various lists of substances that result from the work of the authorities and are published by ECHA on its website.
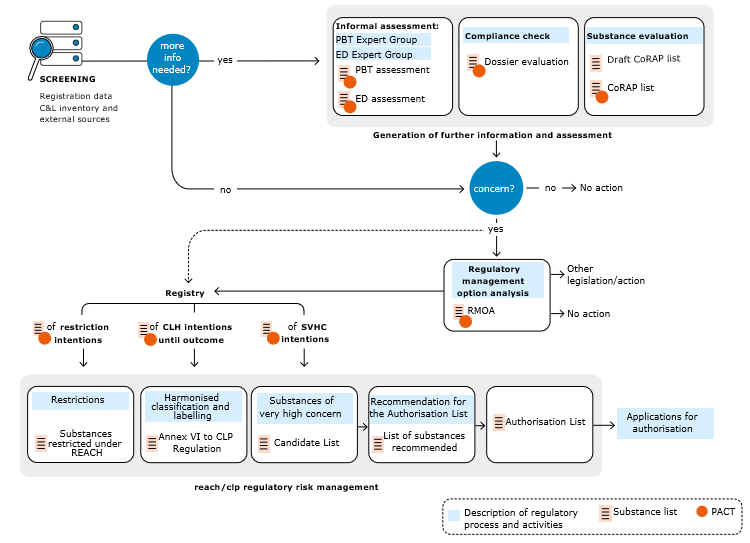 Source: European Chemicals Agency, http://echa.europa.eu/
Source: European Chemicals Agency, http://echa.europa.eu/
It is of little doubt that PACT has increased the transparency and predictability of authorities’ work which leads up to the more formal REACH and CLP processes. The PACT list is updated on a regular basis once the activities concerning a substance are concluded by ECHA.
Suggested reading:
ECHA: Public Activities Co-ordination Tool
ECHA: Substances of potential concern
ECHA: Integrated Regulatory Strategy



